New ADHD Drug Aizhida Offers All-Day Relief for Millions in China
- 23 million children and adolescents in China are affected by ADHD, with a national prevalence of 6.4%.
- Only 500 ADHD-trained physicians are available for a pediatric population of 200 million.
- Aizhida provides up to 13 hours of sustained symptom control with a once-daily dose.
Experts view Aizhida as a significant advancement in ADHD treatment, offering a convenient and effective therapeutic option that addresses critical gaps in care for millions of patients in China.
New ADHD Drug Aizhida Offers All-Day Relief for Millions in China
SHANGHAI, China – January 08, 2026 – China's National Medical Products Administration (NMPA) has officially approved Aizhida, a new once-daily treatment for Attention Deficit Hyperactivity Disorder (ADHD), marking a significant milestone for millions of patients in a country where treatment options have long been limited. The marketing authorization, granted to Shanghai-based Ark Biopharmaceutical Co., Ltd. ("ArkBio"), introduces an innovative therapy designed to provide both rapid and sustained symptom control for individuals aged six and older.
Addressing a Silent Public Health Crisis
ADHD is one of the most common neurodevelopmental disorders, yet in China, it remains a largely unaddressed public health issue. The national prevalence is estimated at 6.4%, affecting a staggering 23 million children and adolescents. Despite these numbers, the path to diagnosis and treatment is fraught with challenges. Studies indicate that over 90% of childhood ADHD cases in China may go undiagnosed, and of those who are diagnosed, only a fraction receive appropriate medical care.
This treatment gap is fueled by a combination of factors. A profound shortage of trained specialists—with estimates suggesting only 500 ADHD-trained physicians for a pediatric population of 200 million—means that specialized care is scarce and concentrated in major urban centers. Compounding the issue are significant sociocultural barriers, including a persistent stigma around mental health and a common misperception among parents that ADHD is a matter of "bad behavior" rather than a treatable medical condition. This often leads to a fear of medication, particularly stimulants, and a delay in seeking effective intervention.
For the few who do receive treatment, the journey is often imperfect. Patients frequently discontinue existing medications due to suboptimal response, inconvenient dosing schedules requiring multiple pills per day, or disruptive side effects. This environment has created a substantial unmet need for new therapeutic options that are not only effective but also convenient and well-tolerated.
A Novel Mechanism for All-Day Control
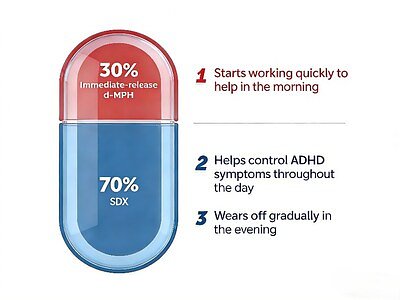
Aizhida enters the market as a first-of-its-kind therapy in China, engineered to overcome the limitations of previous treatments. Its unique formulation combines two forms of dexmethylphenidate, a well-established central nervous system stimulant, into a single daily capsule. Each capsule contains 30% immediate-release dexmethylphenidate (d-MPH) and 70% serdexmethylphenidate (SDX), a prodrug that the body gradually converts into active d-MPH.
This dual-action mechanism is designed for a "rapid onset, full-day coverage" profile. The immediate-release component is absorbed quickly, typically beginning to work within 30 minutes of ingestion to help manage symptoms at the start of the day. Meanwhile, the SDX prodrug is processed slowly in the lower gastrointestinal tract, providing a smooth, extended release of medication that lasts for up to 13 hours. This sustained effect helps children and adolescents maintain focus through the school day, homework, and evening activities, potentially reducing the "rebound effect" or symptom return that can occur as other medications wear off.
This innovative approach, which earned Aizhida a Priority Review designation from the NMPA, has already been validated on the global stage. The same drug was approved by the U.S. Food and Drug Administration (FDA) in March 2021 under the brand name Azstarys. The robust clinical data from a pivotal Phase III trial in Chinese patients further solidified its case, demonstrating statistically significant and clinically meaningful improvements in core ADHD symptoms compared to a placebo.
A Pivotal Moment for Patients and Clinicians
The approval has been met with optimism from leading medical experts in China who have been on the front lines of the country's struggle with ADHD. They see Aizhida not just as another drug, but as a new tool that could fundamentally change treatment strategies.
"ADHD is one of the most common neurodevelopmental disorders in childhood, with potential impact lasting into adulthood, posing long-term challenges to learning, social functioning, and family life," commented Professor Yi Zheng, Chief Expert at Beijing Anding Hospital, Capital Medical University, and a lead investigator of the Aizhida Phase III trial. "While the prevalence in Chinese children is around 6.4%, treatment options—especially innovative ones balancing efficacy and safety—remain insufficient. The approval of Aizhida provides clinicians with a new therapeutic tool."
The convenience of a once-daily dose that provides consistent coverage is seen as a major advantage in improving treatment adherence, a common hurdle for families managing the condition. Professor Jing Liu, Director of the Child Psychiatry Center at Peking University Sixth Hospital and another lead investigator, emphasized the broader context. "Comprehensive intervention is essential for ADHD, with pharmacotherapy being a foundational component," she stated. "The approval of a new drug not only increases options but also prompts deeper reflection on optimizing treatment strategies. We hope the approval of Aizhida will enrich ADHD treatment approaches and contribute to better long-term outcomes."
ArkBio's Strategic Entry into a Shifting Market
For ArkBio, a global biotech company founded in 2014 with a focus on pediatric and respiratory diseases, the NMPA's decision represents a landmark achievement and a crucial strategic victory. The approval allows the company to commercialize Aizhida in one of the world's largest and most underserved healthcare markets. With the ADHD therapeutics market in China projected to grow significantly in the coming years, Aizhida is well-positioned to become a cornerstone treatment.
The successful navigation of the regulatory process also highlights a broader trend within China. The NMPA is increasingly creating expedited pathways for innovative drugs that address urgent public health needs, signaling a welcoming environment for both domestic and international biopharmaceutical companies. ArkBio's ability to generate strong local clinical data for a globally recognized therapy demonstrates a successful model for market entry.
As ArkBio moves into the commercial launch phase, the introduction of Aizhida is expected to reshape the competitive landscape, challenging existing therapies by offering a clear clinical advantage. This development, combined with growing government initiatives to raise mental health awareness, offers a new wave of hope for millions of families, promising a future where children with ADHD have a better opportunity to reach their full potential.
📝 This article is still being updated
Are you a relevant expert who could contribute your opinion or insights to this article? We'd love to hear from you. We will give you full credit for your contribution.
Contribute Your Expertise →