FOLFIRINOX: A Repurposed Chemotherapy Offers New Hope for Biliary Cancer
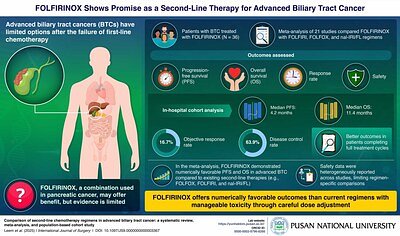
- Median Overall Survival (OS): 11.4 months in the South Korean study cohort, 8.9 months in the broader meta-analysis
- Toxicity: Nearly 40% of patients developed severe neutropenia
- Progression-Free Survival (PFS): 4.2 months in the study cohort
Experts conclude that FOLFIRINOX shows promising efficacy as a second-line treatment for advanced biliary tract cancer, but its high toxicity requires careful patient selection and close monitoring.
FOLFIRINOX: A Repurposed Chemotherapy Offers New Hope for Biliary Cancer
BUSAN, South Korea – November 28, 2025 – In the relentless fight against aggressive gastrointestinal cancers, a potent chemotherapy regimen traditionally reserved for pancreatic cancer is emerging as a potential new weapon against advanced biliary tract cancer (BTC). A recent study from South Korea suggests that FOLFIRINOX could extend survival for patients whose disease has progressed after initial treatment, offering a glimmer of hope in a therapeutic landscape with notoriously few effective options.
This development, stemming from a comprehensive analysis by researchers at Pusan National University and Yonsei University, highlights a critical strategy in modern oncology: repurposing existing, powerful drugs for new indications. However, the promise of extended life comes with a significant trade-off—a high level of toxicity that demands careful management and patient selection, underscoring the complex risk-benefit calculations clinicians and patients must navigate.
A New Look at an Old Foe
Biliary tract cancers, which include malignancies of the gallbladder and bile ducts, are characterized by late diagnosis and aggressive biology. For patients with advanced or metastatic disease, the standard first-line treatment is typically a combination of gemcitabine and cisplatin. While this provides an initial benefit, the cancer almost inevitably progresses, leaving patients and their oncologists with a challenging decision for second-line therapy.
The current options are limited. The landmark ABC-06 trial established FOLFOX (a combination of folinic acid, fluorouracil, and oxaliplatin) as a standard second-line choice, but it offers only a modest survival advantage over supportive care alone. Other regimens like FOLFIRI are also used, but no single option has proven dramatically effective, creating a significant unmet need for more potent therapies.
Into this challenging environment comes the new data on FOLFIRINOX. The South Korean study, published in the International Journal of Surgery, provides compelling evidence by combining two powerful research methods. The team, led by Professor Yun Hak Kim of Pusan National University, analyzed 12 years of real-world data from 54 advanced BTC patients treated with FOLFIRINOX at Yonsei Severance Hospital. They then integrated these findings into a larger systematic review and meta-analysis of 21 relevant global studies. This dual approach provides both a granular look at a single institution's experience and a broad, evidence-based global perspective.
The results were numerically encouraging. In their own patient cohort, researchers observed a median progression-free survival (PFS) of 4.2 months and a median overall survival (OS) of an impressive 11.4 months. The broader meta-analysis reinforced this, showing that FOLFIRINOX achieved a pooled overall survival of 8.9 months, which was numerically superior to other common second-line regimens. "Our findings suggest that FOLFIRINOX may offer a potential benefit as a second-line treatment option for BTC following progression on first-line chemotherapy," concluded Prof. Kim.
The Double-Edged Sword of Potency
While the survival data is promising, FOLFIRINOX is a notoriously difficult regimen for patients to tolerate. Its potency against cancer cells is mirrored by its harsh effects on the body. The study from Pusan National University was transparent about this challenge, reporting that nearly 40 percent of patients developed severe neutropenia—a dangerous drop in a type of white blood cell that is crucial for fighting infection. This level of toxicity often necessitates dose reductions, treatment delays, or the use of supportive medications like granulocyte colony-stimulating factor (G-CSF) to boost white blood cell counts.
This high toxicity profile means FOLFIRINOX is not a one-size-fits-all solution. The study authors caution that the regimen should be reserved for fit patients with a good performance status (ECOG PS 0-1) who can be closely monitored for side effects. For these select patients, the potential survival benefit may outweigh the risks. This reality forces a difficult conversation in the clinic, balancing the desire to extend life against the imperative to maintain the patient's quality of life.
Clinicians are already accustomed to this balancing act. To mitigate the harsh side effects, many cancer centers have adopted modified FOLFIRINOX (mFOLFIRINOX) regimens, which typically involve reducing the dose of certain components or omitting the initial bolus injection of fluorouracil. These modifications have been shown to reduce toxicity while largely preserving efficacy in pancreatic cancer, a strategy that is now being applied in the context of BTC.
The Path to Validation and Future Integration
The study from Pusan National University and Yonsei University provides a strong rationale for considering FOLFIRINOX in advanced BTC, but it is not the final word. The authors rightly emphasize that their findings, based on retrospective data and meta-analysis, must be validated in large, prospective, randomized clinical trials. Such trials are essential to definitively establish FOLFIRINOX's place in the treatment algorithm and to better define the patient populations most likely to benefit.
Some of this work is already underway. The AMEBICA trial, for instance, is evaluating a modified FOLFIRINOX regimen as a first-line treatment, while other Phase II studies have explored it in the second-line setting, with results that echo the Korean findings: promising efficacy coupled with significant toxicity. These ongoing efforts are critical for building the high-level evidence needed to change global treatment guidelines from organizations like the NCCN and ESMO.
Looking ahead, the future of BTC treatment will likely involve more personalized approaches. The study authors suggest that future research could explore combining FOLFIRINOX with immunotherapy or other molecularly targeted drugs. As our understanding of BTC's genetic drivers improves, identifying biomarkers could help predict which patients will respond best to FOLFIRINOX or other therapies, further refining patient selection. For now, this rigorous analysis provides a crucial piece of evidence, giving clinicians a potentially more effective, albeit challenging, option to discuss with patients who have exhausted first-line therapy and are searching for their next line of defense.
