China's Heart-Tech Gambit: Enginprime's OpusOne Challenges Global Giants
A Chinese startup's tiny new heart pump enters human trials, aiming to disrupt a billion-dollar market and solve a massive unmet need in cardiac care.
China's Heart-Tech Gambit: Enginprime's OpusOne Challenges Global Giants
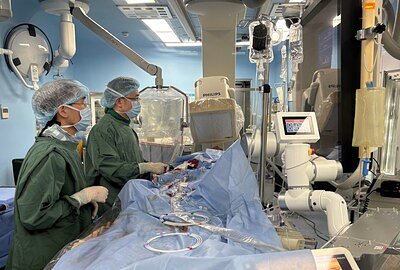
HANGZHOU, China – December 15, 2025 – In the high-stakes world of advanced medical devices, a new contender has emerged from an unexpected corner. Enginprime Medical Inc., a Hangzhou-based startup established just two years ago, has fired a starting pistol in a race long dominated by Western incumbents. The company announced it has initiated the first-in-human clinical study for its OpusOne™ percutaneous ventricular assist device (pVAD), a miniature heart pump designed to support patients through high-risk cardiac procedures.
This is far more than a routine clinical trial announcement. It represents a significant milestone in a market with peculiar dynamics: while over 60,000 pVAD procedures are performed globally each year, not a single device of this kind has yet been approved for use in China. Enginprime, incubated by the venture firm Voyagers Capital, isn't just developing a new product; it's aiming to be the first to unlock a vast, untapped market, potentially reshaping the competitive landscape and signaling a new era for China's domestic MedTech ambitions.
The Engineering Edge: Smaller, Smarter, Safer
The battle for the pVAD market is fought in millimeters and milliliters per minute. These devices are threaded through arteries to the heart to provide temporary mechanical support, augmenting blood flow when the heart is too weak. For years, the primary challenge has been balancing power with invasiveness. Larger pumps provide more flow but risk greater vascular trauma, while smaller devices may not offer adequate support.
Enginprime's OpusOne™ tackles this challenge head-on with a design that appears to synthesize the best features emerging across the industry. Its most striking feature is an ultra-low 8F profile for its catheter. To put this in perspective, the workhorse of the market, Abiomed's Impella CP, requires a much larger 14F sheath. This reduction in size is critical, as it promises to minimize vascular complications—a persistent concern for interventional cardiologists—and potentially expand the eligible patient population to those with smaller or more delicate arteries.
This miniaturization is achieved without sacrificing power. The device boasts an average flow of 4–5 L/min with peaks exceeding 7 L/min, placing it squarely in competition with high-flow systems from market leader Abiomed and other innovators like Magenta Medical and Supira Medical. The key may lie in its foldable, self-expanding impeller, which travels through the artery in a compressed state before deploying to its full size within the ventricle, and its use of an external motor, which removes heat and bulk from the intracardiac component.
Beyond size and power, OpusOne™ integrates advanced safety features. A built-in system for invasive blood pressure monitoring provides real-time hemodynamic data, while a patented perfusion purification system is designed to prevent wear particles from entering the bloodstream—addressing a critical safety concern for any implanted mechanical device. As the study's lead investigator, Dr. Jian'an Wang of SAHZU, noted, "I am impressed by its ability to achieve high flow with such a low-profile design. OpusOneTM's optimization of access and positioning options increases procedural safety."
A Startup's Bold Play in a Billion-Dollar Arena
Enginprime is entering a market projected to exceed $6 billion by 2030, but it's a field dominated by a titan: Abiomed, now part of Johnson & Johnson. For years, Abiomed's Impella has been the default pVAD, building a formidable moat of clinical data, physician training programs, and established hospital contracts. Any new entrant faces the monumental task of not only proving technological superiority but also convincing risk-averse clinicians and administrators to switch.
This is where Enginprime's backing by Voyagers Capital becomes crucial. Voyagers isn't just a source of funding; it incubated the company, leveraging its model of building ventures around breakthrough technologies. This approach provides Enginprime with the strategic runway needed to navigate the long and expensive path to commercialization. By framing OpusOne™ as a "globally competitive, exceptional performance and high reliability, cost-effective" solution, the company is signaling its intention to compete on both innovation and value—a powerful combination in today's healthcare environment.
The competitive strategy appears to be one of targeted disruption. By focusing on a key clinical pain point (vascular access), integrating next-generation safety features, and aiming for cost-effectiveness, Enginprime is creating a compelling argument for adoption. It's a classic "startup versus Goliath" narrative, but one where the startup has a powerful slingshot forged from advanced engineering and strategic venture backing.
Navigating China's MedTech Gauntlet
While OpusOne™ has global ambitions, its initial battleground is China. The lack of any NMPA-approved pVADs presents both a massive opportunity and a significant hurdle. Enginprime has a clear field, but it must also blaze the trail through China's notoriously complex regulatory process for high-risk Class III medical devices. This journey often takes years and requires extensive local clinical trial data, a process the company has just begun.
However, being a domestically incubated company provides a distinct advantage. Beijing's "Made in China 2025" initiative and other policies are actively promoting domestic champions in high-tech sectors, including advanced medical devices. These policies can translate into regulatory fast-tracks, preferential treatment in hospital procurement tenders, and other incentives that foreign firms find difficult to access. Enginprime is perfectly positioned to ride this wave of national industrial strategy.
Success for Enginprime in China would be a landmark event. It would not only provide a vital new therapy for millions of Chinese cardiac patients but also serve as a powerful proof-of-concept for the country's ability to innovate—not just imitate—at the highest levels of medical technology. It would challenge the long-held assumption that breakthrough medical devices primarily originate in the West.
The first-in-human procedure at SAHZU, a top cardiac center, marks a promising start. Dr. Jun Jiang, the study's co-investigator, reported that the initial patient's "chest tightness was significantly alleviated," calling the device's debut a "meaningful advancement in circulatory support." While this is just the first data point in a long validation process, it provides the crucial initial evidence that the engineering concepts behind OpusOne™ translate into tangible clinical benefit. For Enginprime, its investors, and the future of cardiac care in China, this small step represents a giant leap forward.
📝 This article is still being updated
Are you a relevant expert who could contribute your opinion or insights to this article? We'd love to hear from you. We will give you full credit for your contribution.
Contribute Your Expertise →