Able Medical Challenges Metal's Reign in Sternal Closure with PEEK
- PEEK is radiolucent, allowing unobstructed imaging of the sternum, unlike metal implants.
- PEEK's modulus of elasticity is similar to natural bone, potentially reducing stress shielding.
- Able Medical's system is positioned as the first-to-market PEEK rigid fixation system for sternal closure.
Experts in cardiothoracic surgery are likely to view PEEK-based sternal closure systems as a promising advancement over traditional metal implants, offering improved imaging, bone-like flexibility, and potential long-term stability benefits, though further clinical validation is needed.
Able Medical Challenges Metal's Reign in Sternal Closure with PEEK
MARQUETTE, MI – January 19, 2026 – For decades, patients undergoing open-heart surgery have had their sternums wired back together with stainless steel or fixed with titanium plates. Now, a Michigan-based medical device company is set to showcase a technology that could fundamentally change this practice. Able Medical Devices will present its advanced PEEK-based sternal closure system at the upcoming Society of Thoracic Surgeons (STS) 2026 Annual Meeting in New Orleans, positioning the high-performance polymer as a superior alternative to traditional metal implants.
The announcement signals a significant push within the cardiothoracic field towards materials that not only provide stability but also enhance patient recovery and improve post-operative diagnostics. As surgeons and healthcare providers convene from January 29 to February 1, all eyes will be on innovations like Able Medical's, which promise to move beyond simple fixation to a more integrated approach to healing the chest wall.
The PEEK Revolution in Cardiothoracic Surgery
At the heart of Able Medical’s innovation is PEEK (polyether ether ketone), a thermoplastic polymer renowned for its unique combination of strength, biocompatibility, and radiolucency. While new to some aspects of sternal closure, PEEK has a long and successful track record in other medical fields, particularly in spinal and orthopedic implants, where its properties have proven invaluable.
Unlike metal implants, which are visible on X-rays and can create significant artifacts on CT and MRI scans, PEEK is radiolucent. This allows surgeons and radiologists an unobstructed view of the healing sternum, making it easier to monitor bone fusion and detect potential complications like infections or non-union without the visual interference common with metal hardware. This advantage is critical during the fragile post-operative period.
Furthermore, PEEK possesses a modulus of elasticity—a measure of stiffness—that is remarkably similar to that of natural bone. Traditional metal implants, being far more rigid, can cause a phenomenon known as "stress shielding," where the implant bears too much of the body's load, leading to a potential weakening of the underlying bone over time. By flexing in a way that more closely mimics bone, PEEK implants may promote healthier bone remodeling and distribute stress more naturally, potentially leading to greater patient comfort and long-term stability.
"Our team’s collaboration with surgeons and engineers have driven the development of a solution that enhances patient care while simplifying the surgical experience for the cardiothoracic O.R. teams,” said Peter Didyk, SVP & Managing Director at Able Medical, in a recent statement. This collaboration underscores the focus on creating a system that is not just materially advanced but also practically superior in the operating room. Another key benefit is its performance in emergent situations; should a patient require urgent re-entry into the chest, PEEK-based devices can be cut through more easily than titanium plates, saving critical time.
Navigating a Crowded and Innovative Market
While Able Medical has touted its system as a first-to-market solution, it enters a competitive landscape where the shift away from traditional wires toward rigid fixation and advanced materials is already underway. The company’s claim centers on it being the "first-to-market PEEK rigid fixation system," a specific classification that aims to differentiate it from other products.
The sternal closure market is populated by industry giants and specialized innovators. DePuy Synthes, a Johnson & Johnson company, already offers its Sternal ZIPFIX System, which utilizes PEEK implants. Similarly, the Spanish firm Neos Surgery S.L. secured FDA 510(k) clearance for its PEEK-based STERN FIX Sternal Stabilization System as early as 2021. Meanwhile, Zimmer Biomet solidified its market position by acquiring A&E Medical in 2020, gaining a broad portfolio of sternal closure products.
This competitive environment highlights a clear industry trend: a growing consensus that rigid fixation provides superior stability, especially for high-risk patients (including those with obesity, diabetes, or osteoporosis), leading to better healing and a lower incidence of deep sternal wound infections. The introduction of another PEEK-based system from Able Medical reinforces this trend and provides surgeons with more options, driving further innovation and potentially lowering costs over time. The company’s success will depend on its ability to demonstrate clear clinical advantages and surgeon-friendly application for its specific design.
From Regulatory Clearance to Clinical Practice
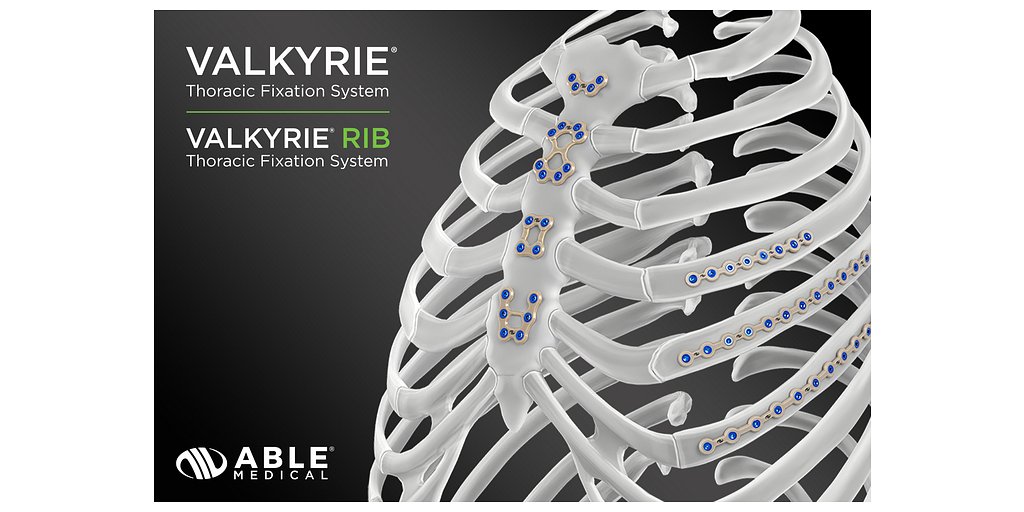
Able Medical is not a newcomer to PEEK-based thoracic solutions. The company has already established a regulatory foothold with its Valkyrie™ Thoracic Fixation System, which received FDA 510(k) clearance in March 2021 for stabilizing chest wall fractures, including sternal fixation. More recently, in January 2024, its Valkyrie RIB System, an extension of the platform, also gained clearance. The system being showcased at STS 2026 appears to be the latest evolution of this PEEK-based platform, specifically optimized and marketed for primary sternal closure.
This existing regulatory foundation provides a strong basis for the company's market entry. However, the ultimate adoption of any new medical device hinges on robust clinical evidence. While Able Medical has emphasized its development process involving surgical teams, the broader medical community will be looking for peer-reviewed data demonstrating improved patient outcomes.
Independent research already provides some support for the material's use in this context. A multicenter retrospective clinical trial comparing PEEK cables to traditional stainless steel wires for sternal fixation found the polymer cables to be effective and easy to implant, with no significant difference in adverse outcomes. Such studies build confidence in the material itself, but surgeons will anticipate device-specific data from Able Medical to validate the performance of its unique rigid fixation design. The STS meeting will serve as a critical platform for the company to begin sharing this information and gather feedback from the surgical community.
The Patient and Surgeon Perspective
Ultimately, the transition to new materials like PEEK is driven by the pursuit of tangible benefits for both the patient in recovery and the surgeon in the operating room. For patients, the advantages of a metal-free alternative are compelling. Beyond the improved imaging capabilities, the bone-like flexibility of PEEK may translate into less post-operative pain and a more comfortable healing process. Patients may feel less of the implant's presence beneath the skin, a common complaint with metal plates.
For surgeons, the appeal lies in a system that is both effective and efficient. The design of modern fixation systems aims to simplify the closure process, reducing time in the operating room. Able Medical's focus on simplifying the surgical experience suggests its system has been engineered for ease of use, potentially reducing the learning curve often associated with new devices.
Despite the benefits, considerations such as cost remain. Advanced polymers and the manufacturing processes for medical-grade implants can be more expensive than traditional materials, a factor that hospital administrators must weigh against the potential for reduced complication rates and improved long-term outcomes. As Able Medical prepares to engage with the cardiothoracic community in New Orleans, it will need to present a comprehensive value proposition that addresses not only the clinical advantages of its PEEK system but also its economic viability in a healthcare system focused on both quality and efficiency. The upcoming showcase will be a pivotal moment in determining if this advanced polymer can truly displace the metal that has held the field together for generations.
