The Unseen Shield: A New Device Tackles a Hidden Healthcare Hazard
Zephyrus Innovations just got FDA clearance for VaporShield, a device protecting nurses from drug exposure. It’s a story of safety, market disruption, and efficiency.
The Unseen Shield: A New Device Tackles a Hidden Healthcare Hazard
VANCOUVER, BC – December 16, 2025
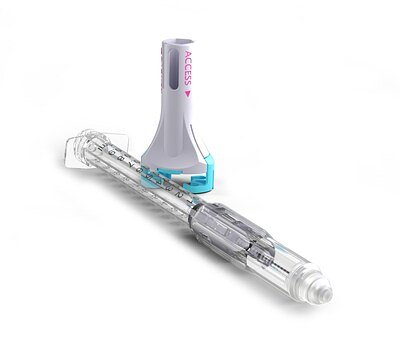
In the world of medical technology, an FDA clearance is always a milestone. But some approvals resonate far beyond the company announcement, signaling a fundamental shift in patient care or, in this case, the protection of caregivers themselves. The recent 510(k) marketing clearance for Zephyrus Innovations' VaporShield™ is one such moment. On the surface, it’s a new Closed System Transfer Device (CSTD). But beyond the launch, it represents a long-awaited solution to a dangerous and often overlooked gap in clinical safety, with the potential to disrupt a multi-billion dollar market.
For years, the healthcare industry has grappled with protecting clinicians from exposure to hazardous drugs like chemotherapeutics and potent biologics. The launch of VaporShield™—the world's first direct injection CSTD designed specifically for subcutaneous and intramuscular routes—isn't just an incremental improvement; it's a direct answer to a silent crisis faced by millions of nurses and pharmacists daily.
The Risk in a Routine Injection
Every day, oncology nurses and other healthcare professionals handle substances capable of causing cancer, reproductive harm, and organ toxicity. The risks of exposure through spills or aerosolization during drug preparation and intravenous (IV) administration are well-documented. This led to the creation of CSTDs and the implementation of stringent USP guidelines, which mandate their use to contain hazardous drugs.
However, a critical vulnerability remained. While IV administration was covered, the routine act of giving a subcutaneous (under the skin) or intramuscular (into the muscle) injection lacked a purpose-built, compliant solution. Clinicians were often left with a workflow that, despite best efforts, could not guarantee a fully closed system from drug vial to patient. This meant the potential for splattering, spraying, or aerosolization of potent drugs remained a persistent threat.
"You follow every protocol, you wear your PPE, but there's always a nagging thought about the transfer, the connection, the disposal," explained a veteran oncology nurse from a major metropolitan hospital. "A tiny, invisible droplet aerosolized during the process is all it takes. The long-term consequences of these micro-exposures are what keep us up at night." VaporShield™ directly confronts this gap. By providing an end-to-end closed system, it is designed to meet the stringent containment requirements of USP for these specific injection types, a feat no other device has accomplished in a single, integrated system.
A Trifecta of Innovation
The significance of VaporShield™ lies not in a single feature, but in its holistic approach to a complex problem. Zephyrus Innovations has engineered a device that combines three critical safety and efficiency elements.
First and foremost is its function as a true closed system. The device creates an airtight seal from the moment it attaches to the drug vial, through the drawing of the medication, and all the way to the injection itself. This design prevents the escape of drug vapors or aerosols, effectively shielding the clinician and the environment from contamination.
Second, it integrates the proven technology from Zephyrus's previously cleared Aeroject™ syringe: auto-retractable needlestick prevention. Immediately after the dose is delivered, the needle automatically and smoothly retracts back into the syringe barrel, rendering it safe and preventing accidental needlestick injuries. This dual-threat protection—against both chemical exposure and bloodborne pathogens—is a powerful combination.
Third, the device boasts an ultra-low dead space design. This engineering detail has profound implications. It minimizes the amount of medication left in the syringe hub after an injection, which not only allows for more accurate patient dosing but also significantly reduces the waste of incredibly expensive hazardous drugs. For a healthcare system perpetually focused on cost-containment, this efficiency can translate into substantial financial savings.
Disrupting a Billion-Dollar Market
While the impact on clinician safety is paramount, the business implications of VaporShield™'s arrival are impossible to ignore. The global CSTD market is a lucrative and rapidly growing space, projected to surpass $2.7 billion by 2030, with the United States accounting for nearly 40% of sales. This market is currently dominated by established giants like BD, ICU Medical, and Equashield.
Zephyrus Innovations, a private and comparatively newer player, is executing a classic disruption strategy. Instead of competing head-on in the crowded IV CSTD space, it identified a critical, unserved niche and built a superior solution for it. By being the 'world's first' to offer a direct injection CSTD for subQ/IM routes, Zephyrus has created a defensible market position built on a clear regulatory and safety need.
Guy Reynolds, Executive Chairman of Zephyrus, highlighted this game-changing potential in the company's announcement. "The seriously negative health impacts caused by exposure to hazardous drugs are well documented but unfortunately, there has been no device that fully protects healthcare workers giving these drugs via subcutaneous or intramuscular administration, until now," he stated. "There is now a significant opportunity to protect millions of healthcare workers around the world."
This strategic focus, building upon the foundation of its earlier Aeroject™ safety syringe, demonstrates a clear vision: innovate where the need is greatest and the existing solutions are weakest. For investors and industry watchers, Zephyrus is now a company poised to capture a meaningful share of a burgeoning market by solving a problem its larger competitors had not.
Beyond Hospital Walls
The impact of this innovation extends beyond the immediate benefit of protecting workers in cancer centers. It aligns perfectly with one of the most significant trends in modern medicine: the shift toward decentralized care and the 'Hospital at Home' model. As more complex treatments, including the administration of biologics and other specialty drugs, move out of the hospital and into outpatient clinics or even patients' homes, the need for simple, foolproof safety devices becomes paramount.
VaporShield's single-system simplicity and integrated safety features make it an ideal enabling technology for this transition. It provides a level of containment and protection that can ensure both clinician and caregiver safety, regardless of the setting. As healthcare continues its decentralization, moving from the centralized hospital to the community and the home, the demand for technologies that are not only effective but also inherently safe for a wider range of users will only intensify. Innovations like VaporShield are not just protecting today’s clinicians; they are laying the groundwork for a safer, more distributed model of care for tomorrow.
📝 This article is still being updated
Are you a relevant expert who could contribute your opinion or insights to this article? We'd love to hear from you. We will give you full credit for your contribution.
Contribute Your Expertise →