New Light-Activated Patch Seals Brain Tears in Seconds, Aims for Safer Surgery
- 10% of spinal and cranial surgeries experience durotomy (tears in the dura mater).
- The patch achieves adhesion strength up to ten times higher than commercial tissue adhesives.
- The patch swells less than 200%, minimizing mass effect risks.
Experts view this light-activated patch as a significant advancement in neurosurgery, offering a safer, faster, and more reliable method for sealing dural tears compared to existing solutions.
New Light-Activated Patch Seals Brain Tears in Seconds, Aims for Safer Surgery
BUSAN, South Korea – January 16, 2026 – By Christine Carter
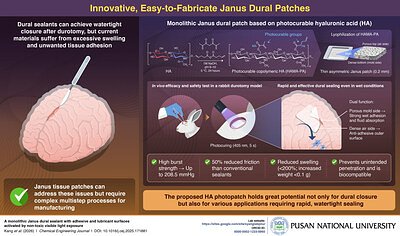
A breakthrough in medical biomaterials from South Korean researchers promises to make one of the most common and dangerous complications in neurosurgery significantly easier to manage. A team at Pusan National University has developed an innovative, light-activated patch that can create a rapid, watertight seal over tears in the dura mater—the protective membrane surrounding the brain and spinal cord—in a matter of seconds, potentially preventing life-threatening postoperative complications.
The novel patch, detailed in the Chemical Engineering Journal, uses a modified form of a natural biopolymer and a unique two-sided design to overcome the critical limitations of current surgical closure methods. This development could represent a major leap forward in patient safety and surgical efficiency, with a clear path toward clinical use already underway.
Addressing a Critical Neurosurgical Challenge
Durotomy, an accidental tear of the dura mater, is a frequent and unwelcome event in operating rooms, occurring in up to 10% of spinal and cranial surgeries. When this delicate membrane is breached, it can lead to the leakage of cerebrospinal fluid (CSF), a severe complication that can cause debilitating headaches, delayed healing, and increase the risk of dangerous infections like meningitis. Achieving a durable, watertight closure is therefore paramount for a successful surgical outcome.
For decades, the gold standard for dural repair has been meticulous suturing. However, this technique is time-consuming, technically demanding, and can be impractical in hard-to-reach areas. Furthermore, each needle puncture creates a new potential leak point. To address these issues, surgeons often turn to adjuncts like synthetic patches and tissue adhesives.
While helpful, these existing solutions are not without their own significant drawbacks. Many glue-based sealants swell considerably upon contact with bodily fluids, creating a dangerous “mass effect” that can compress sensitive brain or spinal cord tissue. They can also cause unwanted adhesion to surrounding structures, leading to future complications, or prove too brittle to withstand the pressure within the skull. The search for an ideal dural sealant—one that is strong, biocompatible, non-swelling, and easy to apply—has been a long-standing goal in the field.
The Science of a 'Two-Faced' Solution
The Pusan National University team, led by Professor Seung Yun Yang from the Department of Biomaterials Science, has tackled these challenges with an elegant and effective design. Their innovation is a monolithic “Janus” patch, named after the two-faced Roman god, which features two distinct surfaces with different properties.
"Made from natural biopolymer hyaluronic acid, our dural patch provides strong wet adhesion, along with a lubricating surface that prevents unwanted tissue adhesion, after exposure to non-toxic visible light," explained Prof. Yang in the university's announcement.
The foundation of the patch is hyaluronic acid (HA), a substance naturally found in the human body and widely used in medical products for its exceptional biocompatibility and lubricating qualities. The researchers chemically modified the HA with light-sensitive groups, creating a photocurable material. This solution is then processed to form a thin patch, approximately 0.2 mm thick, with a dense, polymer-rich surface on one side and a porous, lower-concentration surface on the other.
When placed over a dural tear, the dense surface faces the tissue. A five-second exposure to low-energy visible light activates the material, creating an incredibly strong bond. Laboratory tests revealed an adhesion strength up to ten times higher than that of commercially available tissue adhesives, capable of withstanding high burst pressures. Simultaneously, the porous outer surface acts as an anti-adhesive, lubricating barrier that prevents the patch from sticking to other tissues, while also efficiently absorbing excess fluid.
Crucially, the patch demonstrated minimal swelling—less than 200%—and a negligible increase in weight, effectively eliminating the risk of mass effect that plagues many current sealants. Its high stretchability and flexibility allow it to conform perfectly to the contours of the brain and spinal cord, ensuring a complete seal. In preclinical tests on a rabbit durotomy model, the patch provided rapid and effective dural closure without causing any observable damage to the surrounding skull, dura, or sensitive brain tissue.
A Clear Path from Lab to Commercialization
This promising technology is not destined to remain in the laboratory. The intellectual property has been transferred to SNvia Co., Ltd., a South Korean biotech company spun out of Pusan National University with expertise in photocurable polymers. SNvia has already established large-scale manufacturing facilities for the photocrosslinkable hyaluronic acid, positioning it to rapidly scale up production.
The company is moving swiftly toward clinical application. Nonclinical studies are scheduled to conclude in the first half of 2026, with plans to submit a medical device clinical trial application to South Korea's Ministry of Food and Drug Safety (MFDS) within the same year. This collaboration between a leading research university and an agile biotech firm exemplifies a growing trend in South Korea's innovation ecosystem, which is increasingly adept at translating academic discoveries into market-ready medical solutions.
While the path to regulatory approval in major global markets like the United States and Europe is rigorous and lengthy, the strong safety profile of hyaluronic acid and the compelling preclinical data provide a solid foundation for future submissions.
Beyond Brain Surgery: A Platform for Future Medicine
The implications of this research extend far beyond the neurosurgical suite. The underlying technology—a highly biocompatible, light-activated, and strongly adhesive biomaterial—is a versatile platform with vast potential. Professor Yang notes that its ability to bond strongly to wet tissues opens the door to a wide range of applications in regenerative medicine and drug delivery.
This photocrosslinkable hyaluronic acid could be used to create advanced drug-delivery patches that adhere securely to internal organs, or as a bio-ink for 3D printing complex, cell-laden constructs to serve as scaffolds for tissue engineering. The development of artificial tissues and organs could also benefit from a material that can be precisely shaped and cured in place. As research continues, this simple yet powerful innovation born from the need for a better surgical sealant may prove to be a foundational technology for the next generation of medical treatments.
📝 This article is still being updated
Are you a relevant expert who could contribute your opinion or insights to this article? We'd love to hear from you. We will give you full credit for your contribution.
Contribute Your Expertise →