New Gene Therapy for Dry AMD Completes Key Human Safety Trial
- 200 million people globally affected by Age-related Macular Degeneration (AMD)
- $27.5 billion estimated market for AMD treatments by 2031
- 6 patients treated in Phase I trial of PST-611, with no prohibitive safety signals reported
Experts view PST-611 as a promising, disruptive alternative to current dry AMD treatments, with its novel iron dysregulation target and innovative delivery method offering potential for long-lasting therapeutic effects.
A New Attack on Blindness: PulseSight Completes Key Safety Trial for Novel Dry AMD Gene Therapy
PARIS, France – January 22, 2026 – In a significant step forward for the millions battling irreversible vision loss, Paris-based PulseSight Therapeutics has announced the completion of patient dosing in its Phase I clinical trial for PST-611. The novel gene therapy candidate is being developed to treat Geographic Atrophy (GA), the advanced and debilitating form of dry Age-related Macular Degeneration (AMD), a condition for which effective, well-tolerated treatments remain a critical unmet need.
The completion of this first-in-human safety and tolerability study marks a crucial milestone for a therapy that employs a unique strategy to combat the disease. Unlike existing treatments, PST-611 targets the fundamental biology of iron-induced cell death in the retina. With data from the trial slated for presentation at the prestigious 2026 ARVO Annual Meeting in May, the ophthalmology community is watching closely.
The Science of Sight: Targeting Iron Dysregulation
Age-related Macular Degeneration is the foremost cause of central vision loss in the elderly, affecting an estimated 200 million people globally. It robs individuals of the sharp, straight-ahead vision necessary for reading, driving, and recognizing faces. While the 'wet' form of AMD has several treatment options, the more common 'dry' form has been a formidable challenge for medicine. As dry AMD progresses to Geographic Atrophy, regions of retinal cells waste away, leading to permanent blind spots and a profound loss of quality of life.
Recent scientific research has increasingly pointed to a culprit in the progression of dry AMD: the dysregulation of iron. While essential for cellular function, an excess of free, unregulated iron in the retina can trigger a cascade of toxic effects, including chronic inflammation and severe oxidative stress. This ultimately leads to a form of programmed cell death known as ferroptosis, which is believed to be a key driver in the death of the light-sensing photoreceptors and the supportive retinal pigment epithelium (RPE) cells.
PST-611 is a first-in-class candidate designed to intervene directly in this process. It is a non-viral gene therapy that delivers a DNA plasmid encoding human transferrin, the body's natural iron-transporting protein. The therapy's core principle is to restore the eye's ability to manage iron safely. By increasing the local production of transferrin, PST-611 aims to bind the excess toxic iron, preventing ferroptosis and protecting the delicate retinal cells from destruction. Preclinical studies in animal models have already demonstrated that this approach can protect photoreceptors and RPE cells, preserving visual function.
A New Delivery Paradigm: The Eye as a Biofactory
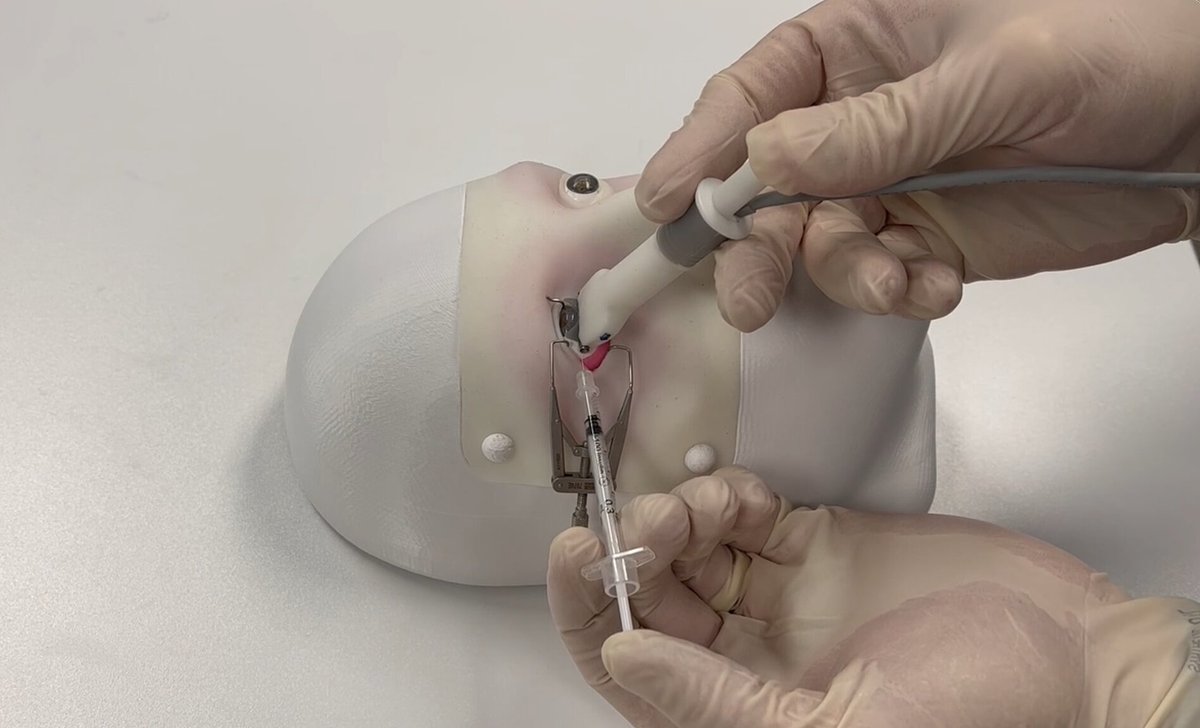
Beyond its novel biological target, PST-611 is distinguished by its innovative delivery method. Current treatments for GA, which target a different biological pathway known as the complement system, require frequent and burdensome injections directly into the vitreous of the eye, often on a monthly or bi-monthly basis. This treatment regimen poses a significant challenge for an elderly patient population.
PulseSight Therapeutics is pioneering a different approach. Its platform uses a minimally-invasive procedure involving electro-transfection to deliver the DNA plasmids into the eye's ciliary muscle. This muscle, located near the front of the eye, is transformed into a sustainable 'biofactory.' Once programmed, its cells begin to express and secrete the therapeutic transferrin protein, which then distributes throughout the retina to perform its protective function. This method is designed to provide long-lasting therapeutic effects, potentially requiring re-treatment only once every four to six months, a dramatic improvement over the current standard of care.
Professor Francine Behar-Cohen of the Cochin Hospital in Paris, a pioneer of the electro-transfection technology and an investigator in the trial, expressed her enthusiasm for the candidate. "Late-stage dry AMD/GA is a progressing disease that leads to vision loss and for which we have no therapeutic options for our patients," she stated. "Based on its mechanism of action and thanks to the innovative delivery technology, PST-611 has potential to become a major treatment option for these patients."
Navigating the Billion-Dollar Blind Spot
The market for AMD treatments is vast, estimated to reach $27.5 billion by 2031. The recent approval of the first-ever drugs for GA—the complement inhibitors Syfovre and Izervay—was a landmark moment, but their adoption has been tempered by limitations. While they have shown a modest ability to slow the growth of GA lesions, they have not yet demonstrated a clear benefit in preserving vision. Furthermore, the frequent injection schedule and safety concerns, including a potential increased risk of conversion to wet AMD, have highlighted the urgent need for alternative and more durable therapies.
PST-611 enters this competitive landscape not as an incremental improvement, but as a disruptive alternative. By targeting an upstream driver of the disease (iron dysregulation) and offering a significantly less burdensome delivery schedule, PulseSight's therapy could carve out a unique and valuable position. The company, backed by investors including Pureos Bioventures, ND Capital, and Korea Investment Partners, is strategically positioned to address a clear gap in the market.
The Path Forward: From Safety Data to Efficacy
The now-completed Phase I study, conducted in Paris and Grenoble, successfully treated six patients across two different dose levels. Its primary goal was to establish the safety and tolerability of PST-611, a critical first step for any new human therapy. The successful completion of dosing suggests no prohibitive safety signals emerged, paving the way for the next stage of development.
"We are very pleased to have completed the enrolment of the patients in the phase I trial," said Dr. George Weissgerber, PulseSight‘s Chief Medical Officer. "This trial marks the first step of PST-611 clinical development and provides the foundation to build upon for the phase IIa trial we are already preparing to further demonstrate PST-611’s potential."
The upcoming presentation at the ARVO 2026 meeting will be the first public look at the human data. Researchers and investors will be keenly interested in the detailed safety profile, including the frequency and severity of any adverse events. While the trial was not designed to measure efficacy, any exploratory data or biomarkers suggesting that PST-611 is having its intended biological effect—or even hinting at a stabilization of vision or lesion growth in these first few patients—would generate significant excitement and provide crucial validation for this promising new approach to saving sight.
📝 This article is still being updated
Are you a relevant expert who could contribute your opinion or insights to this article? We'd love to hear from you. We will give you full credit for your contribution.
Contribute Your Expertise →