AI Enters the Cleanroom: DeepHow's PharmaCloud Aims for Compliance
- Over 80% of production deviations in pharmaceutical manufacturing are attributed to human error. - PharmaCloud claims to save manufacturers between $300,000 and $500,000 annually through a 10-20% improvement in process consistency. - Onboarding a new operator for GMP-critical roles can require 200 to 300 hours of training per person.
Experts would likely conclude that DeepHow's PharmaCloud represents a significant advancement in bridging the gap between AI innovation and regulatory compliance in pharmaceutical manufacturing, offering a potentially transformative solution for reducing human error and operational costs.
AI Enters the Cleanroom: DeepHow's PharmaCloud Aims for Compliance
DETROIT, MI – January 19, 2026 – In a move that could reshape frontline operations in one of the world's most regulated industries, AI-solutions provider DeepHow today announced the launch of PharmaCloud. The new platform offers a GMP-compliant cloud environment designed to standardize training, guide complex tasks, and verify operator work in pharmaceutical and medical device manufacturing using artificial intelligence.
The launch directly confronts a fundamental challenge that has long stymied technological progress on the factory floor: the tension between adopting powerful new tools and adhering to stringent regulatory mandates. While AI holds the promise of dramatically improving consistency and reducing errors, the validation and compliance hurdles in pharmaceutical production have often made its deployment a high-risk endeavor.
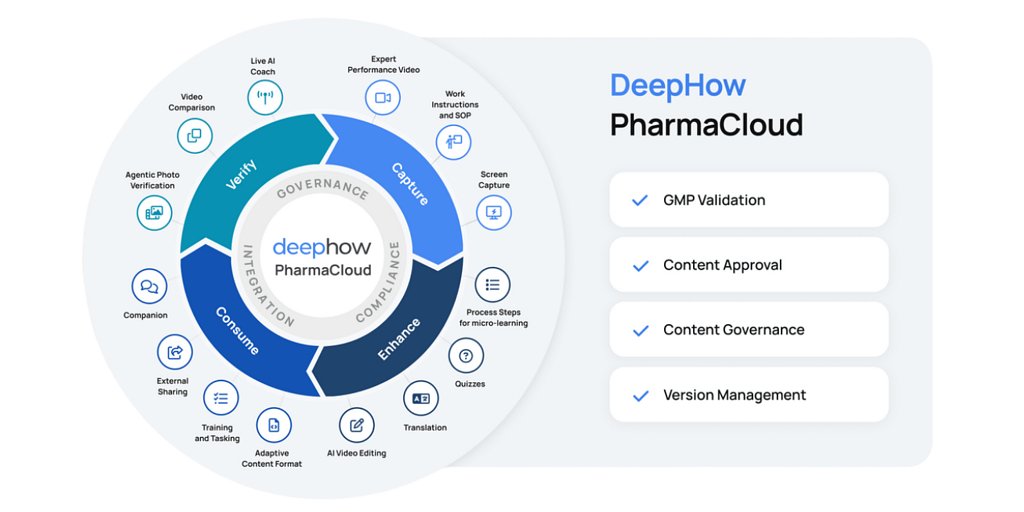
PharmaCloud is engineered to dismantle that barrier. By delivering its AI-powered operational knowledge platform within a pre-validated, audit-ready framework, DeepHow aims to provide manufacturers with a clear path to modernization without introducing compliance friction.
“Pharmaceutical manufacturing should not have to choose between innovation and audit readiness,” said Sivakumar Lakshmanan, CEO of DeepHow, in a statement accompanying the announcement. “PharmaCloud provides a GMP-compliant foundation to deploy advanced AI directly at the point of work, ensuring operators are trained, guided, and verified as they perform mission-critical tasks.”
Tackling the Human Element in a Regulated World
At the heart of pharmaceutical manufacturing lies a persistent vulnerability: process variability. Industry data suggests that over 80% of production deviations are attributed to human error, often stemming from inconsistent training, complex Standard Operating Procedures (SOPs), and the gradual loss of institutional knowledge as veteran operators retire. These seemingly small inconsistencies can have enormous consequences, leading to costly batch failures, product recalls, and intensive regulatory investigations.
The challenge is compounded by the increasing complexity of modern biologics and sterile products, which demand highly nuanced manual processes. Onboarding a new operator for these GMP-critical roles can be a resource-intensive affair, sometimes requiring 200 to 300 hours of training per person.
DeepHow's platform targets this issue by transforming how knowledge is captured and disseminated. The system uses AI to analyze video recordings of expert operators at work, automatically breaking down the footage into clear, step-by-step interactive instructions. This accelerates the creation of training materials and makes expert knowledge searchable and accessible on demand.
PharmaCloud takes this a step further by bringing AI directly to the point of execution. The platform features live AI coaching and visual verification capabilities that use computer vision and Visual Language Models (VLMs). As an operator performs a task, the system can confirm that critical steps are completed correctly, flag missing actions or incorrect sequences, and ensure adherence to the validated SOP in real-time. This creates a digital co-pilot for the operator, designed to minimize error and reinforce best practices with every cycle.
Building an Audit-Ready Digital Foundation
For years, the pharmaceutical industry has approached cloud-based solutions with caution, concerned about data security, system validation, and maintaining control over software updates. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) enforce strict rules for electronic records and computerized systems, such as FDA 21 CFR Part 11, which governs data integrity, audit trails, and electronic signatures.
PharmaCloud was designed from the ground up to operate within this framework. The platform's claim of being "GMP-compliant" rests on a suite of built-in features for governance, traceability, and control. All content, from training videos to procedural guides, is subject to version management and formal approval workflows. This ensures that operators are always working from the most current, validated procedure.
Every action, update, and training record generates a corresponding electronic record, creating a comprehensive and immutable audit trail that satisfies the ALCOA++ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) for data integrity. AI-driven verification of tasks automatically documents proof of correct execution, providing manufacturers with robust evidence for auditors and regulatory reporting. This digital-first approach to documentation aims to significantly reduce the time and effort required to prepare for audits.
The Economics of AI-Driven Operations
The operational improvements promised by PharmaCloud are tied to a compelling economic case. According to DeepHow's own ROI modeling, even a modest 10-20% improvement in process consistency can save a typical manufacturing site between $300,000 and $500,000 annually in reduced deviation costs alone. By accelerating onboarding and reducing the need for constant retraining, the platform addresses a significant operational expense fueled by workforce turnover.
Beyond cost reduction, the system is positioned as a driver of efficiency and throughput. By ensuring consistent execution and providing real-time guidance, manufacturers can expect more stable product quality and greater reliability in meeting production schedules. The platform is also designed for integration, capable of connecting with existing enterprise systems like Learning Management Systems (LMS), Quality Management Systems (QMS), and Manufacturing Execution Systems (MES). This allows for the seamless flow of training records and procedural updates without disruptive manual reconciliation, positioning PharmaCloud as a specialized intelligence layer within a company's existing digital ecosystem.
By unifying training, execution, and verification on a single compliant platform, DeepHow is proposing a new operating model for regulated manufacturing. It envisions a future where the adoption of advanced AI does not force a trade-off with compliance but instead becomes a primary tool for achieving it, setting a potential new benchmark for how technology and regulation can work in concert to ensure product quality and patient safety.
